Model or part number: 7395-13
About this product
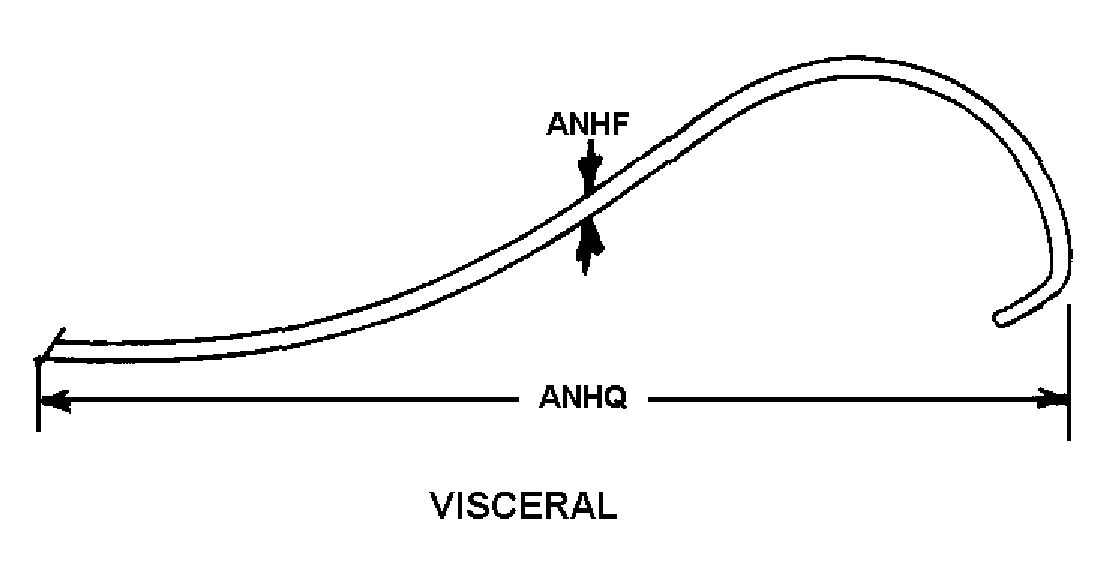
6f softouch internal mammary catheter (ima style);length of tip 3.3cm;recommended guide wire 0.97mm

Factory New, New Material, New Surplus, Overhauled, Serviceable, Repairable, and AS Removed / Used material will be accompanied by a Certificate of Conformance, and any other documents/trace when applicable. We have a 7 year record retention.

We are committed to quality and follow an AS9120 quality management system. AeroBase Group is HAZMAT certified and registered with DDTC,
ITAR, and Aviation Suppliers Association (ASA). All products are 100% inspected.
Quality Commitment »

Delivery of IN-STOCK orders ship same-day when order is placed before 2:00 PM EST.
Delivery dates for NON-STOCK items are estimates based on current backlog and are subject to confirmation at the time of order.
A component of a catheter and needle unit, intravenous. It is a flexible, homogenous, hollow, cylindrical item, having a funnel or luer end, suitable for introduction into a vein. It is nontoxic and free from tissue reaction, and capable of insertion through a hypodermic needle of specific size. For items without funnel or luer end, see tube, intravenous.
Bougies, Catheters, Drains and Sondes and Parts and Accessories
Medicinal Equipment
Syringes, Needles, Catheters, Cannulae and The Like Used In Medical, Surgical Or Veterinary Sciences
| Code | Definition |
|---|---|
| FN | Factory New. Parts manufactured by the OEM and includes manufacturer certification. |
| NE | New. Parts manufactured by the OEM. May include manufacturer certification or company certification. |
| NS | New Surplus. New material item purchased from excess inventory. |
| OH | Overhauled. Product has been rebuilt and tested in accordance with the manufacturer's specifications by a repair shop (MRO). |
| SV | Serviceable product. |
| AR | As Removed. Product removed from aircraft or platform with no guarentee on the functionality of the part. |
| RP | Repairable. Product can be repaired by a MRO and be given a FAA 8130 or EASA Form 1 certification. |
| Part Number | RNVC | RNAAC | DAC | CAGE |
|---|---|---|---|---|
| 7395-13 | 2 | 9Z | 4 | 0VS26 |
| Name | No. Items | |||
|---|---|---|---|---|
| [You must be logged in to use lists] | ||||